You are viewing 1 of your 1 free articles. For unlimited access take a risk-free trial
Infection and skin health in sport: don’t play dirty!

Despite the inevitable preoccupation with coronavirus, good old skin infections courtesy of pathogens such as MRSA are still all too common among athletes. PP explains why, and how the correct hygiene measures can ensure athlete health – especially in team and close-contact sports
For most sportsmen and women, the most commonly associated health risks of exercise involve sporting injuries. However, what’s far less appreciated is the health risks from infection. Although coronavirus has a high media profile at the moment, a major risk for younger athletes is skin infection, which occurs as a result of close contact - for example in team sports or the use of community facilities such as gyms and changing rooms. This is undesirable because poorly-managed infectious disease may result in detrimental consequences to the health of athletes and also their chances of sporting success. To make matters worse, inaccurate diagnoses may lead to regional epidemics, as well as substantial amounts of missed time from sport.Type and incidence of infection
What kinds of infections can occur as a result of close or cross-contact? A large 2012 meta-study reviewed all the literature published over the previous 20 years on the subject of infections within the sporting environment(1). It found the following:- The most marked risk is skin infection with Methicillin-Resistant community acquired Staphylococcus Aureus (MRSA), which was reported in 27% of the articles. This was followed by Tinea corporis and capitis (14% of articles), and leptospirosis (12% of articles);
- The risk of blood-borne infection was low, and articles reporting these kinds of infections were rare (just 4%). However, the authors concluded that the risk of diseases with respiratory transmission (eg measles, meningococcal meningitis etc) must also be taken into account;
- The effect of physical activity on the immune system depends on the type and duration of the work out: the evidence suggests that it seems to be beneficial for a workout of a moderate intensity, and deleterious for a sustained acute work out, or a period of intensive training. These periods of protection or susceptibility to infections are described as ‘open window’ and ‘J-curve’ (see figure 1).
Figure 1: The J-shaped curve of immunity and training

At very low levels of training, immunity is average. As training load increases (and fitness improves), immunity is typically enhanced. However, at very high training loads, immunity declines markedly and infection risk rises to above average. Infection is most likely to occur in the hours following an intense training session when immunity is most compromised – the so-called ‘open window’.
Accurate data on sport-related infection is hard to come by; infection rates will vary depending on the type of sport, the particular environment in which an athlete trains/competes and importantly, the extent of reporting by athletes and coaches alike. One study estimated that of all sports-related conditions, skin-related infectious diseases accounted for 8.5% of these among high school students, rising to 20.9% of all conditions among college students(2).
Breaking these figures down by type, the authors found that about half of the skin infections affected the head, face or neck, probably as a result of direct skin-to-skin contact with infected opponents. As for organisms responsible, the study found that among high-school athletes, the figures were as follows: bacteria 30%; herpes viruses 20%; tinea fungi 20%. The figures for collegiate athletes were herpes viruses 47%, impetigo,37%, tinea fungi 7%, cellulitis 6% and MRSA 3%. In particular, other studies have reported that herpes gladiatorum accounts for around 20% of all sports-related skin infections(3,4).
The incidence of contracting a skin infection from direct contact while competing against an infected athlete is high - estimated at around 33%(5). Data from US wrestling tournaments held in Minnesota between 1997 and 2006 revealed an incidence of skin infections of between 2.5-3.7 per 100 individual competitors, with the highest incidence reported in urban areas(6,7). Meanwhile, the National Collegiate Athletic Association’s statistics on wrestling injuries from 1988-2004 reveal an incidence of skin infections of around 0.98 of 1000 athlete exposures (an exposure defined as equivalent to one practice or game)(8).
Bacterial infection and MRSA
Any infection arising from participation in sport (whether of the skin or otherwise) is clearly unwelcome. For young athletes however, it is perhaps bacterial, and particularly MRSA infection, that is of the greatest concern. This is partly due to its resistance to treatment (see box 1) and partly because of the complications that may follow an MRSA infection. Studies show that community-acquired (CA) MRSA colonized individuals and their close contacts are more likely to develop skin and soft tissue infections(9). Among non-institutionalized adults in the United States, routine testing (of the nasal passages) showed that around 1.5% of the population are asymptomatic carriers of MRSA(10). However, in certain populations, these rates can be much higher. Rates of 3% have been reported among US soldiers(11), 7% among US graduates(12) and 23% among US college athletes(13) – almost 1 in 4! These findings appear to tie in with other studies that indicate the incidence of CA-MRSA has increased recently in people who are generally perceived as healthy such as athletes and soldiers(14-16).Community-acquired MRSA infections of the skin and soft tissues are becoming more common, and the incidence has been reported in association with various sports seasons. Reports of football teams with CA-MRSA indicate that increased exposure to training rooms and equipment (eg gyms, changing rooms, treatment rooms) is associated with increased cases of CA-MRSA among players and cosmetic body shaving among athletes has been associated with increases rates of infection(17-19).
For example, in a team of players that tested negative for nasal MRSA at the beginning of a season, five members later were identified as having CA-MRSA(20). Another study showed that the number of nasal-colonized student athletes varied across the season as a function of the intensity of training(21). It’s unclear why some colonized individuals develop MRSA infection and others do not but one theory is that colonization sites away from the nose (eg as the genitalia - not tested in these studies) may play a more important role.
Risk factors for MRSA (the 5 Cs)
Given that most sportsmen and women are fitter and healthier than their sedentary counterparts, the obvious question is why should they be at a greater risk of MRSA infection? The ‘5Cs’ scheme shown in figure 2 provides a partial explanation. The main risk factors for acquiring MRSA and going on to develop an active infection are a crowded environment, lack of cleanliness (the environment or individual), the use of contaminated items, physical contact with others, compromised skin integrity.Figure 2: The 5Cs as risk factors for MRSA

It’s not difficult to imagine a scenario where for example, sportsmen and women training together for or competing in team sports involving close contact and who suffer minor skin abrasions and cuts in the process will tick at least three or four of the five risk boxes! One study of US football players revealed that the majority of MRSA infections occurred early in the season and among those players experiencing the most direct contact (eg linemen and linebackers)(22). Other high high-risk sports for MRSA include basketball, fencing, rugby, volleyball, weight lifting, and wrestling(22).
Box 1: The rise and rise of MRSA
In 1929, Alexander Fleming discovered that a secretion of the mold called Penicillium had the ability to kill a number of bacteria, including some staphylococci. He called the filtrate of a broth of this culture ‘penicillin’ – the first ever antibiotic. However, within a year after the introduction of penicillin, some strains of staphylococcus aureus (S aureus) had already become resistant and S aureus went on to develop resistance to other antibiotics. By the 1950s, the first epidemics of penicillin-resistant staphylococci were being reported in Europe and North America, only a decade after the use of postoperative antibiotics became commonplace.By 1959, penicillin-resistant strains of S aureus were already considered pandemic so methicillin was introduced as an antibiotic. However, as early as 1961, S aureus had begun developing resistance to methicillin – a resistance that quickly spread worldwide - and is now considered endemic within most hospitals (hospital acquired [HA] MRSA).
Over the years since, S aureus has continued to evolve and become resistant to ever more powerful antibiotics, a phenomenon that is increasingly worrying to health professionals. More than 50% of MRSA strains are now resistant to drugs such as macrolides, lincosamides, fluoroquinolones, and aminoglycosides; and 30% are resistant to trimethoprim-sulfamethoxazole. Likewise, Vancomycin was one of the few remaining medications to control difficult MRSA; however, vancomycin-resistant MRSA is now a reality(23).
HA-MRSA has also transcended the confines of health care institutions to produce infection in healthy members of the community – so-called ‘community acquired (CA) MRSA. Although MRSA is transmitted more easily in the community, it has generally remained more susceptible to a broader range of antibiotics(24). Unfortunately however, multidrug resistance in CA-MRSA has been detected and as community and hospital strains intermingle and patients and community members bring these strains into the hospital and vice versa, there is growing concern that highly-virulent community strains that affect healthy individuals will become less susceptible to antibiotics.
MRSA identification, treatment and prevention
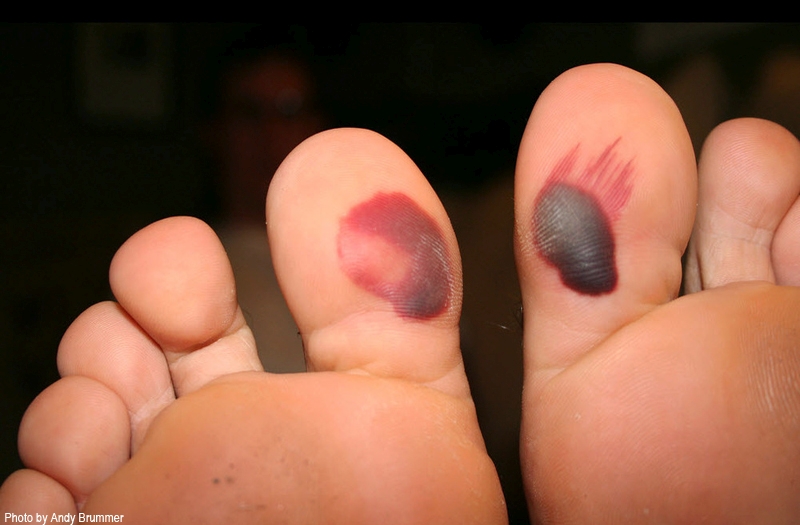
An MRSA infection of the skin often mimics other lesions so proper precautions and clinical suspicion are warranted by athletes and those who care for athletes. The majority of MRSA infections are cutaneous affecting the skin), involving cellulitis, an abscess, or both. Pain and pus production at the site of infection accompanied by inflammation and swelling are characteristic of MRSA infections and cutaneous MRSA lesions will frequently occur at the site of an abrasion or cut, even if the injury is mild (eg athletes with artificial turf abrasions or who have used cosmetic shaving can develop MRSA skin infections). One problem that can potentially delay the identification and treatment of an MRSA infection is that the athlete may mistakenly believe the lesion to be an insect bite due to visual similarities in the early stages of infection.When it comes to treatment for MRSA infections of the skin, the gold standard is lesion incision and drainage followed by oral antibiotic treatment after abscess culture identification. MRSA infections that fail initial treatment may require multidrug therapy, such as the combination of vancomycin with one or more additional antibiotics. Prevention of course is better than cure and in this respect, coaches and healthcare providers for athletes should consider prevention strategies. These include limiting as far as possible the risk factors outlined in the ‘5Cs’ scheme and immediately applying topical antibiotic ointment to any cuts abrasions that occur in the course of training or competition.
Another option is to consider eliminating any MRSA colonization of the athlete in the first place because studies show that existing colonization quadruples an athlete’s risk of infection(25). However this course of action is far from straightforward. For starters, there are currently no standardized guidelines in the literature for MRSA colonization eradication in athletes. Secondly, the decolonization treatment protocols used are controversial, typically requiring a 7-day course of topical antibiotic ointment applied to the nostrils (bacitracin, mupirocin etc) combined with skin washes (eg chlorhexidine gluconate 4%, silver sulfide 2% and tea tree 10%) and systemic (oral) antibiotics. Studies show that this kind of regime has less than 40% compliance, which means there’s the real possibility that it may help develop further resistance in MRSA-colonized athletes(26,27).
Practical steps for athletes and their coaches/trainers/physiotherapists
In this article, we’ve discussed the characteristics, incidence, risk factors and treatment options associated with MRSA infection in the context of sport. Hopefully, it will be apparent from the foregoing that by far the best strategy for managing MRSA - as with coronavirus - is prevention. What does this boil down to in practice? Here are some practical recommendations for athletes and for those who care for or treat athletes. Bear in mind too that many of these MRSA-prevention recommendations will also reduce the risk of contracting other infectious diseases too, including coronavirus!- *For healthy athletes without signs and symptoms of MRSA infection, basic hygiene practice is the key to prevent infection. Hands should be washed regularly and thoroughly with soap and warm water. If this is not possible, hand washing can be replaced by alcohol-based hand rubs but only if the hands are not visibly soiled (in which case soap and water are required).
- *To reduce the risk of cross infection, sportsmen and women should never share personal items such as towels, razors, facecloths, dirty clothes or used athletic gear. Athletes should also be discouraged from sharing protective gear, such as cycling helmets, gloves etc and even from sharing bar soap. They should also endeavor to ensure that their personal items such as day-to-day clothing, bedding, towels and work/study areas are kept as clean as possible.
- For team athletes, any team kit should be freshly laundered and the clubhouse environment kept as clean as possible.
- If an athlete has a skin infection of any kind (remember MRSA should always be suspected), he or she should be discouraged from trying to self-treat by popping, draining, or lancing it, all of which can spread the risk of infection. Instead, they should seek the advice of their health care provider as soon as possible.
- Athletes with known MRSA infections should not use swimming pools, including therapy/spa whirlpools, unless the pool water is regularly changed after use.
- In the gym, shared training equipment, such as weight machines and benches, should be cleaned with disinfectant on all surfaces where there’s a possibility of the skin touching the equipment. Surfaces such as floors, mats, and doors should also be thoroughly cleaned on a regular basis.
- Where MRSA infection exists, wounds should be completely covered with a bandage until they are healed, and any wound containing pus should be covered with a clean, dry bandage to prevent the spread of infection.
- Disposable applicators or tongue depressors should be used when applying medications and ointments, and they should not be reintroduced to containers after use.
- Any health professionals, sports clinicians or coaches participating in the changing of wound dressings should wash their hands thoroughly immediately afterwards.
- When MRSA is confirmed, disqualification from sports participation should include practice, competition, and any other form of direct skin contact with members of the team/squad until the athlete can be shown to be clear of infection and is cleared by the appropriate medical team.
- Med Mal Infect. 2012 Nov;42(11):533-44
- Am J Sports Med. 2008;36(1):57-64
- Dermatol Nurs. 2008;20(1):39-44
- Adolesc Med. 2001;12(2):305-322
- Med Sci Sports Exerc. 2003;35(11):1809-1814
- Curr Sports Med Rep. 2008;7(6):323-327
- Clin J Sport Med. 2007;17(6):478-480
- J Athl Train. 2007;42(2):303-310
- Lancet 2010;375(9725):1557-68
- J Infect Dis 2008;197(9):1226-34
- Clin Infect Dis 2004;39(7):971-9.
- Clin Lab Sci 2009;22(3):176-84
- Arch Pediatr Adolesc Med 2010;164(7):615-20
- J Am Acad Dermatol 2007;56(1):1-16 [quiz 7-20]
- Proc Natl Acad Sci U S A 2009;106(14):5883-8
- Clin Infect Dis 2004;39(10):1446-53
- N Engl J Med 2005;352(5):468-75
- J Athl Train 2006;41(2):141-5
- J Community Health Nurs 2009;26(4):161-72
- Clin J Sport Med 2009;19(6):498-501
- Arch Pediatr Adolesc Med 2010;164(7):615-20
- Med Sci Sports Exerc. 2008;40(8):1362-1367
- Clin Infect Dis 2005;40(4):562-73
- Lancet 2010;375(9725):1557-68
- Am J Med. 2008;121(4):310-315.
- Cochrane Database Syst Rev. 2003;(4):CD003340
- Pediatr Infect Dis J. 2005;24(9):841-843
Newsletter Sign Up
Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Newsletter Sign Up
Coaches Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Keep up with latest sports science research and apply it to maximize performance
Today you have the chance to join a group of athletes, and sports coaches/trainers who all have something special in common...
They use the latest research to improve performance for themselves and their clients - both athletes and sports teams - with help from global specialists in the fields of sports science, sports medicine and sports psychology.
They do this by reading Sports Performance Bulletin, an easy-to-digest but serious-minded journal dedicated to high performance sports. SPB offers a wealth of information and insight into the latest research, in an easily-accessible and understood format, along with a wealth of practical recommendations.
*includes 3 coaching manuals
Get Inspired
All the latest techniques and approaches
Sports Performance Bulletin helps dedicated endurance athletes improve their performance. Sense-checking the latest sports science research, and sourcing evidence and case studies to support findings, Sports Performance Bulletin turns proven insights into easily digestible practical advice. Supporting athletes, coaches and professionals who wish to ensure their guidance and programmes are kept right up to date and based on credible science.