You are viewing 1 of your 1 free articles. For unlimited access take a risk-free trial
Stress fracture: why athletes need to bone up on nutrition

Stress fractures are one of the most common and potentially serious overuse injuries reported in athletes, both elite and recreational. With this in mind, Andrew Hamilton looks at recent evidence linking stress fractures and nutritional status…
Although stress fractures are most commonly associated with modern athletes undergoing hard training, the earliest reports of this injury were documented in the late 19th and early 20th century among soldiers engaged in military training. During World War II in particular, increasing numbers of military studies described march fractures in bones of the lower extremities, primarily the tibia and femur(1).In more recent years however, experience has taught us that almost any athlete or exerciser who performs frequent and repetitive activity may develop a stress fracture. Among this population, the most vulnerable individuals are likely to be those whose sports or pastimes involve repetitive weight-bearing activities such as running. As in soldiers, the most common sites for developing a stress fracture are the lower extremities(2-4) – in distance runners for example, the tibia is the most vulnerable location for a stress fracture(5-7).
However, stress fractures are not limited to the lower body; studies have shown that stress fractures can occur in the ribs as a result of heavy rowing training(8) and the spine of fast bowlers in cricket. In the case of rowers for example, a meta-study pooled the findings from a number of previous studies on rib fracture in rowers and came up with some startling results(9). For example, the incidence of rib stress fracture in elite rowers was as high as 16.4% (almost 1 in 6), while even in university and junior rowers, the risk was still significant at 2% and 1% respectively.
What causes stress fracture?
Stress fractures (sometimes dubbed ‘hairline fractures’) occur essentially as the result of an incomplete adaptation to long-term mechanical stress. This stress may involve very significant loads (as is often the case in athletes) or loads that are relatively light but which an individual is unaccustomed to. This means that stress fractures can also occur in those who are relatively sedentary but who have recently taken up a new activity – for example someone who has been fairly inactive but has undertaken a sudden burst of exercise.In terms of the underlying physiology, stress fractures are thought to occur as a result of an imbalance in the process of osteoclastic bone breakdown and new bone reformation by osteoblasts. Repetitive heavy loads that stress the bones cause osteoclastic resorption of bone. If, over a prolonged period of time, this process outstrips the osteoblastic formation of new bone, the result is a weakened bone that is vulnerable to injury.
There’s also evidence that altered muscle biomechanics (eg fatigue or muscle imbalance) can also play a role in the occurrence of stress fractures. That’s because the forces of each loading are absorbed and distributed by the bone/muscle system. However, if the muscles are unable to function optimally (eg they become fatigued), they lose some of their ability to absorb and distribute these forces, which further loads the skeletal system.
Risk factors for stress fracture
Apart from repetitive heavy loading during exercise, what are the main risk factors for stress fracture? In a detailed and systemic review of the scientific literature, researchers identified a number of intrinsic and extrinsic risk factors(10). These are shown below in table 1. However, what’s notable by its absence from this table is any reference to dietary factors. This is perhaps surprising when we know that stress fracture results from a remodeling imbalance in bone metabolism and that optimal bone metabolism is intimately linked to the plentiful presence of nutrients such as calcium and vitamin D.Table 1: Risk factors for stress fracture(10)
Intrinsic risk factors
Female sex (especially where a history of amenorrhea, menstrual irregularity, etc is present)Increased age
Non-white ethnicity
Anatomic factors (including high-foot arches, knock knees, high quadriceps angles & leg length discrepancies
Low bone density
Lower fitness (including lower aerobic fitness, flexibility, strength and muscle endurance)
Sedentary lifestyle
Tobacco use
Previous history of injury
Extrinsic risk factors
Type of activity/sportPoor equipment (eg poorly cushioned shoes)
Use of orthotic inserts
Training environment (eg predominantly tarmac)
One difficulty facing researchers in this field are the ethical considerations; given the health ramifications, it would be very difficult to conduct an intervention study that reduced vitamin D and calcium intake in subjects to see whether the incidence of stress fracture rose! Moreover, when stress fracture is reported, any investigation into the dietary habits of the subject prior to the fracture tends to be retrospective, which is obviously prone to considerable inaccuracy. Then of course, there’s the multifactorial nature of this injury, which makes it difficult to determine the exact importance of diet as a risk factor.
The calcium and vitamin D connection
In more recent years however, it has become apparent that nutritional status does indeed play a role in determining the risk of stress fracture, both in athletes and in military personnel. One reason for our improved understanding in this area is the steadily growing interest in sports nutrition generally, combined with improved methods of measuring the status of nutrients such as vitamin D. This has led to a growing body of published evidence on the importance of nutritional status for reducing the risk of stress fracture.For example, a randomized trial in female military recruits demonstrated that calcium and vitamin D supplementation reduced the incidence of stress fractures and also that young female runners suffered a reduced incidence of stress fractures and increased their bone mineral density with increased dietary calcium intake(11). Findings from these studies together suggest female athletes and military recruits who consumed greater than 1500 mg of calcium daily exhibited the largest reduction in stress fracture injuries.
Meanwhile, another study into 125 young (18-26 years), female cross-country runners looked at the nutrients, foods, and dietary patterns associated with stress fracture risk and changes in bone mineral density (BMD) over a 2-year period(12). To do this, the BMD of the spine, hip, and total body was measured annually by dual x-ray absorptiometry; the 17 instances of stress fractures that occurred were recorded on monthly calendars and confirmed by radiograph, bone scan, or magnetic resonance imaging. The main finding of the study was that higher intakes of calcium, skimmed milk, and dairy products were associated with lower rates of stress fracture. Indeed, each additional cup of skimmed milk consumed per day was associated with a 62% reduction in stress fracture incidence! Moreover, higher intakes of calcium, vitamin D, skimmed milk and dairy foods were associated with significant gains in hip BMD.
It’s not just women who may be at increased risk of stress fracture when diet is poor or sub-optimum. A very large Finnish study looked into the association between the blood concentrations of vitamin D and the incidence of stress fracture in 756 young male military recruits(13). At the start of military service, researchers measured blood vitamin D concentrations together with the weight, height, body mass index (BMI), muscle strength, and 12-minute running performance of the recruits. At the end of the study period, data from the recruits who had suffered stress fracture (22 or 2.9%) were compared to those who had remained free of stress fracture. The results showed that:
- *Those who had suffered stress fractures had a significantly lower blood level of vitamin D compared to those who did not.
- *There was no significant association between BMI, age or smoking and stress fracture.
Stress fracture: the risk of being female
Female athletes, along with their coaches and physiotherapists, should be aware that females have a greater incidence of stress fractures than males, something that has also shown to be true in military populations. There are a number of reasons why this appears to be the case. These include differing bone anatomy, lower aerobic capacity, smaller muscles (with less shock absorption capacity) and (often) a poorer diet.The data for this increased risk is quite convincing. For example, one meta-study pooled data from 11 studies focusing on military populations and 10 studies on athletes(16). It found that in both populations, females had higher incidence of stress fractures, with an incidence of approximately 3% and 9.2% overall for males and females respectively in military populations. Among athletes, the incidence was approximately 6.5% and 9.7% for male and female athletes respectively. It’s not all bad news for women though as this study also showed that both female recruits and athletes with normal weight and BMD were less likely to develop stress fractures - showing that gender, while a factor, is less important than the overall physical condition of an individual.
Calcium and vitamin D status in sportsmen and women
Given that there appears to be a profound link between the incidence of stress fracture and sub-optimum intakes of calcium and vitamin D, the obvious question is how likely is it that you may be at risk of a less than ideal intake? This is difficult to assess accurately without the input from a qualified nutritionist. Also, to complicate matters further, your vitamin D status is not just dependent on diet, but also exposure to bright sunshine (see box ‘Sunshine and vitamin D). However, the evidence from studies carried out on sportsmen and women suggests that these two nutrients are frequently a cause for concern, especially in the case of vitamin D during winter months.Sunshine and vitamin D
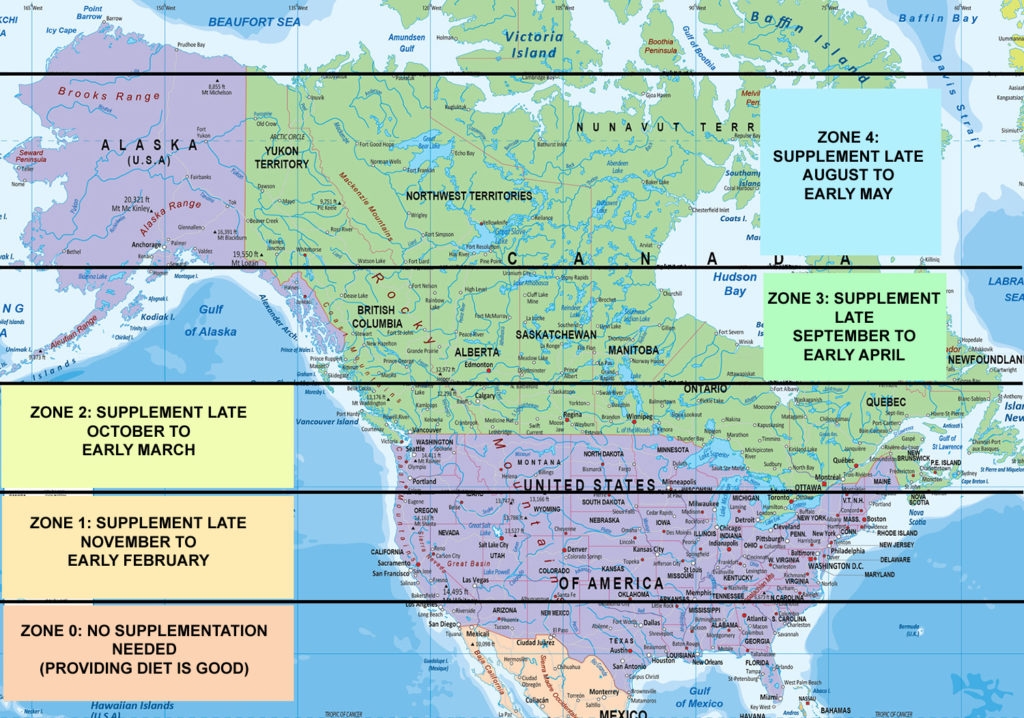
Vitamin D can be synthesized from a natural substance in the body (derived from cholesterol and called 7-dehydrocholesterol) when skin is exposed to sunlight. However, it’s important to note that it’s the UVB rays in sunshine that are needed for this reaction to occur. When the sun is high in the sky, for example during summer months or all year round in the tropics, the sunlight contains sufficient UVB to enable the synthesis of vitamin D. However, when the sun is low in the sky, its rays have to pass through a greater volume of atmosphere to reach the surface, significantly attenuating the UVB content.Indeed, studies have shown that from November through to February, when human skin is exposed to sunlight on clear sunny days at latitudes of around 42oN (the same latitude as cities such as Boston, Rome and Marseilles), the UVB content of that sunshine is so low that no vitamin D can be synthesised even around midday when the sun is highest in the sky(17). This means vitamin D supplementation is required. Move up to around 52oN (for example London, Amsterdam and Berlin) and vitamin D synthesis in the skin becomes impossible from October right through to March! Figure 1 shows the relationship between vitamin D synthesis and latitude for North America.
Figure 1: Latitude and vitamin D supplementation requirements for N America
Vitamin D
Taking vitamin D first, a study by researchers from Israel (a sunny country!) looked at the vitamin D status of 98 Israeli athletes and dancers and found that no less than 73% of the subjects were vitamin D insufficient (which they defined as a blood level of less than 30 nanograms (ng) per millilitre (ml) of blood)(18). And while the insufficiency rates were higher among indoor sports, the rates for outdoor sportsmen and women were still 40%! Given that Israel is a sunny country, these results are surprising and suggest vitamin D deficiency could be even more widespread at more northern latitudes.Meanwhile, a US study looked at how vitamin D levels in 41 athletes from Carolina (12 indoor, 29 outdoor) varied through the seasons(19). In addition, bone density was measured by dual energy x-ray absorptiometry, and illness and injury were documented as part of their routine care. These researchers used 40ng per ml as the cut-off for optimal status and found that, 75.6%, 15.2%, and 36.0% of athletes had an optimal status in the autumn, winter, and spring, respectively. They also found that the low concentrations in the spring were correlated with frequency of illness and recommended vitamin D supplementation during the winter to prevent seasonal decreases.
Further evidence comes from Australia, where it used to be assumed that all young athletes have good vitamin D levels. However, when eighteen female gymnasts aged 10-17 years were assessed for vitamin D status and dietary calcium intake, fifteen were found to have levels below current recommended vitamin D guideline for optimal bone health - 75 nmol/L - and six had vitamin D levels below 50 nmol/L(20).
Numerous other studies suggest vitamin D insufficiency is rife among athletes. These include studies on elite Canadian female footballers(21), Spanish professional basketball players(22) and young Spanish swimmers(23). These findings shouldn’t surprise us however as there’s plenty of evidence that (especially in northern latitudes), sub-optimum vitamin D levels are widespread among the general population. For example, a 2010 German study concluded that vitamin D levels were sub-optimum in nearly half of the general German population and an additional 15-30% were actually vitamin D deficient – ie only a quarter of the population had adequate vitamin D levels(24)!
Calcium
The optimal intake of calcium intake to compensate for skeletal calcium losses and for the prevention of osteoporosis and fractures has been much debated and remains unclear. A good example of this is the wide range of daily calcium recommendations for individuals older than 50 years, which have also changed over time. At present, these are 700mgs in the UK, 800mgs in Scandinavia, 1200mgs in the US and 1300mgs in Australia and New Zealand! Moreover, unlike vitamin D, calcium intakes have attracted much less attention by researchers in recent years.Despite this however there’s still plenty of evidence to suggest that some sportsmen and women may not be consuming optimum amounts. For starters, just by performing a few calculations using the UK Food Standard Agency’s own data it’s possible to demonstrate that anybody consuming less than 1,700 calories per day (for example an athlete trying to lose body fat or maintain a low weight) will struggle to obtain optimum amounts of calcium from their diet, regardless of how carefully balanced the diet.
There are also a number of more specific studies showing that calcium insufficiency can be a real problem among some athletes. For example, when researchers studied the dietary habits of 72 elite female Australian athletes, they found that 24% of them failed to meet the estimated average requirement for calcium(25). Meanwhile, the study on Australian gymnasts above also found that thirteen of the gymnasts also had daily dietary calcium intakes below the daily recommended intake for their age(20). Similar findings of calcium shortfall have been found in female Korean Judo athletes(26), teenage female competitive figure skaters during the competitive season (where 44% of the skaters had intakes less than two thirds of the recommended daily allowance for calcium)(27), US elite female national figure skaters(28), Brazilian adventure racers(29) and Japanese male collegiate footballers(30).
Practical recommendations
Given all this information, what’s the best advice for those who have sportsmen and women at risk of stress fracture (or with a history of stress fracture) in their care? Here are some recommendations that can help reduce the risk:- Emphasize the importance of both calcium-rich and vitamin D-rich foods in the diet. For vitamin D: oily fish such as salmon, mackerel, herring, sardines, pilchards etc are excellent sources; for calcium: milk, yoghurt, cheeses, nuts and seeds should all be consumed regularly.
- Bear in mind seasonal fluctuations in vitamin D status; athletes living and training at northerly latitudes will probably benefit from routine supplementation from October to March (blood tests are readily available that can identify a sub-optimal vitamin D status – ie below 75ng/L).
- Encourage some regular sun exposure to boost vitamin D levels; the latest thinking is that 15 minutes per day in strong sunshine without sun cream is ideal (cover up after that though).
- Be aware that young female athletes are at increased risk of stress fracture, especially when (all too often) dietary habits are less than optimal.
- Take all the usual precautions to ensure a) that exercise loading is increased only very gradually and b) that your athletes allow adequate time for rest and recovery.
- Finally, try and ensure that your athletes’ footwear is up to the job and is assessed regularly for its ability to dissipate shock.
- Am J Surg 1946;71:222–32
- Exerc Sports Sci Rev 1989;17:379–422
- Am J Sports Med 1987;15:46–58
- Orthopedics 1991;14:1089–95
- J Sports Med 1974;2:189–98
- Exerc Sports Med 1980;6:50–9
- Acta Orthop Scand 1978 ;49:19–27
- Skeletal Radiol. 2007 Oct;36(10):951-4
- Sports Med. 2011 Sep 28
- Epidemiol Rev (2002) 24 (2): 228-247
- PM R. 2010 Oct;2(10):945-9
- PM R. 2010 Aug;2(8):740-50
- J Bone Miner Res. 2006 Sep;21(9):1483-8
- Med Sci Sports Exerc. 2008 Nov;40(11 Suppl):S609-22
- J Pediatr Orthop. 2010 Jun;30(4):339-43
- Mil Med. 2011 Apr;176(4):420-30
- J Clin Endocrinol Metab. 1988 Aug;67(2):373-8
- Int J Sport Nutr Exerc Metab. 2008 Apr;18(2):204-24
- Med Sci Sports Exerc. 2011 Feb;43(2):335-43
- Clin J Sport Med. 2008 Mar;18(2):159-61
- Int J Sport Nutr Exerc Metab. 2011 Dec;21(6):507-14
- Nutr Hosp. 2011 Oct;26(5):945-51
- J Strength Cond Res. 2011 Apr;25(4):1126-33
- Mol Nutr Food Res. 2010 Aug;54(8):1164-71
- Int J Sport Nutr Exerc Metab. 2010 Jun;20(3):245-56
- Nutrition. 2002 Jan;18(1):86-90
- J Am Diet Assoc. 2002 Mar;102(3):374-9
- Int J Sport Nutr. 1999 Dec;9(4):345-60
- Nutrition. 2007 May;23(5):404-11
- Asia Pac J Clin Nutr. 2009;18(3):344-50
Newsletter Sign Up
Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Newsletter Sign Up
Coaches Testimonials
Dr. Alexandra Fandetti-Robin, Back & Body Chiropractic
Elspeth Cowell MSCh DpodM SRCh HCPC reg
William Hunter, Nuffield Health
Keep up with latest sports science research and apply it to maximize performance
Today you have the chance to join a group of athletes, and sports coaches/trainers who all have something special in common...
They use the latest research to improve performance for themselves and their clients - both athletes and sports teams - with help from global specialists in the fields of sports science, sports medicine and sports psychology.
They do this by reading Sports Performance Bulletin, an easy-to-digest but serious-minded journal dedicated to high performance sports. SPB offers a wealth of information and insight into the latest research, in an easily-accessible and understood format, along with a wealth of practical recommendations.
*includes 3 coaching manuals
Get Inspired
All the latest techniques and approaches
Sports Performance Bulletin helps dedicated endurance athletes improve their performance. Sense-checking the latest sports science research, and sourcing evidence and case studies to support findings, Sports Performance Bulletin turns proven insights into easily digestible practical advice. Supporting athletes, coaches and professionals who wish to ensure their guidance and programmes are kept right up to date and based on credible science.












